› MBiotech’s Biopharmaceuticals (BioPh) curriculum comprises both required courses, as well as elective courses. Our curriculum map was developed to provide a visual overview of the BioPh course curriculum, detailing not only all of the courses offered but also the topics covered in each course. { or jump to DHT Curriculum | This page last updated: Nov 2025 }

› Curriculum
The MBiotech Program provides an intensive series of courses that provide a solid foundation in the biotechnological space, delivered in two parallel streams. These courses encompass both the scientific and business realms, with overlapping content designed to reinforce key sector-specific knowledge sets and to provide students with a broad interlocking mosaic of concepts.
The core courses for both fields cover related content including business courses, the internship requirements and the Biopartnering seminar series. The remaining content is presented in different courses for each stream to reflect different emphases, as follows—
- The Biopharmaceuticals (BioPh) stream teaches stages of drug development, drug and medical device regulation (with a drug emphasis), clinical trial design and drug action through the molecular biology laboratory and courses in biotechnology in medicine and biomaterials and protein chemistry theory.
- The technical training between the fields is different with BioPh students learning laboratory technique in two courses (Molecular Biology Laboratory and Biomaterials & Protein Chemistry Laboratory) while DHT students learn programming and advanced statistics (Coding in R Language; Data Science in Health I and Data Science in Health, Part II).
› Credit Requirements
Students enrolling in the Biopharmaceuticals specialisation of MBiotech are required to complete 9·5 graduate course credits over a 24-month period on a full-time basis. These 9·5 credits comprise the following—
› 8 Science courses (0·5 credits each, for a total of 4·0 credits)
› 4 Business courses (0·5 credits each, for a total of 2·0 credits)
› 2 Work Term courses (1·0 credit each, for a total of 2·0 credits)
› Electives (1·5 credits)
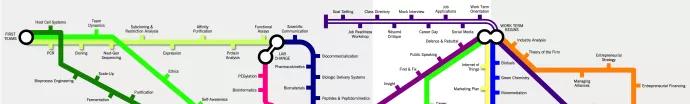
› Our Required Courses(R)
| › YEAR 1 BioPh |
Seminar Series (BTC16x0)
BTC1600H› Biopartnering I › { Sep-Dec }
Science Series (BTC17x0)
BTC1700H › Molecular Biology Laboratory › { May-Jul }
BTC1710H › Biomaterials & Protein Chemistry Theory › { Jul-Aug }
BTC1730H › Modern Data Analysis in Structural Biology and Drug Discovery › { Jul-Aug }
Hybrid Series (BTC18x0)
BTC1800H › Biotechnology in Medicine › { Sep-Oct }
BTC1810H › Biotechnology & Drug Manufacturing › { May-Aug }
BTC1820H › Biotechnology in Agriculture & Natural Products › { Oct-Dec }
Work Term Series (BTC19x0)
BTC1900Y › Work Term I › { Jan-Apr }
Business Series (BTC20x0)
BTC2000H › Effective Management Practices › { May-Dec } Y Course
BTC2010H › Fundamentals of Managerial Concepts › { Sep-Dec }
BTC2020H › Society, Organisations & Technology › { Oct-Dec }
| › YEAR 2 BioPh |
Seminar Series (BTC16x0)
BTC1610H › Biopartnering II › { Sep-Dec }
Work Term Series (BTC19x0)
BTC1910Y › Work Term II › { May-Aug }
Business Series (BTC20x0)
BTC2030H › Management of Technological Innovation › { Jan-Apr }
› Our Electives(E)
| › YEARS 1 & 2 BioPh |
Hybrid Series (BTC18x0)
BTC1896H › Technology & Cognitive Performance › { Sep-Dec }
Work Term Series (BTC19x0)
BTC1920Y › Work Term III › { Sep-Dec }
Special Topic Series (BTC21x0)
BTC2100Y › Thesis Project in Biotechnology
BTC2110H › Supervised Study › { Jan-Apr }
BTC2120H › Decision Analytics in Business, Healthcare & Management › { Jan-Apr }
› Course Descriptions
— All mandatory courses (but not electives) offered in the BioPh stream are described in this section.
BTC1700H › Molecular Biology Laboratory {Year 1}

Session: Summer
Instructor: Leigh Revers
Credits: 0·5 R
Course Description:
This laboratory-based course introduces fundamental experimental techniques commonly used in biomedical research and provides ‘hands-on’ experience working with nucleic acids and proteins over an intensive six-week schedule. Students receive a practical overview of key protocols over the first week and are provided with same-day, interactive technical demonstrations in a fully equipped ‘wet’ laboratory. This is followed by an extended research assignment in which students work in teams towards expressing and isolating a biomedically relevant, recombinant protein. Teams must design an appropriate research strategy, conduct experiments, collect and analyse data and submit their product with a final report to meet a tight deadline. The course concludes with a final presentation seminar day.
BTC1710H › Biomaterials & Protein Chemistry Theory {Year 1}
Session: Summer
Instructor: Cynthia Goh
Credits: 0·5 R
Course Description:
This course is designed to enable students to gain a more in-depth appreciation and understanding of the application of materials science and protein chemistry to the field of biotechnology. We delve into advanced drug delivery and therapeutic strategies, biomaterials in medicine, pharmacology and drug discovery. We also consider new disruptive technologies as case studies for life science biotechnology students.
BTC1730H › Modern Data Analysis in Structural Biology and Drug Discovery {Year 1}
Session: Summer
Instructor: Aziz Abu-Saleh
Credits: 0·5 R
Course Description:
BTC1730H provides hands-on training in computational methods used in modern structural biology and drug discovery, leveraging both the high-performance computing clusters and cloud-based environments such as Google Colab. Students learn to work across multiple platforms to run sequence analysis (BLAST), protein structure prediction using AI-based tools (e.g., AlphaFold, RoseTTAFold), molecular visualization and analysis with (PyMOL), cheminformatics toolkits (RDKit), molecular docking (AutoDock Vina and GNINA), and molecular dynamics simulations. The course emphasizes building integrated computational pipelines, performing data analysis, and executing reproducible workflows. Students work collaboratively in teams reflecting real-world biotech, pharmaceutical, and academic research settings. Students are also evaluated through weekly hands-on assignments, a midterm, a team-based final project presentation, and a final research paper demonstrating a complete computational drug discovery workflow.
BTC1810H › Biotechnology & Drug Manufacturing {Year 1}
Session: Summer
Instructor: Tim Lee
Credits: 0·5 R
Course Description:
Biotechnology & Drug Manufacturing is a half-credit course that introduces students to some of the key aspects of the biopharmaceutical process, with special emphasis on the biotech sector. The course focuses on the fundamental role played by corporate entities in the development of new therapeutic drugs in a highly regulated business environment. Topics covered include biopharmaceutical manufacturing, regulatory approval for drug products and medical devices, setting regulatory standards, quality-by-design, cGMP compliance, risk management and root cause analysis.
BTC2000H › Effective Management Practices {Year 1}

Sessions: Summer & Fall Y Course
Instructor: Eva McLellan & Fatima Nishan
Credits: 0·5 R
Course Description:
This course introduces students to the basic skills and concepts needed to become an effective member of an organisation. It focuses on (1) team working skills, (2) fundamental managerial skills, and (3) career management skills. The course is participative in its design and requires students to apply the material in the course. It provides the first opportunity for a team approach to problem solving and will provide a realistic preview of the work place.
This course will be used to define and organise groups of students who will work in teams to complete the subsequent laboratory modules.
BTC1800H › Biotechnology in Medicine {Year 1}
Session: Fall
Instructor: Jayson Parker
Credits: 0·5 R
Course Description:
This course will introduce students to the development of a wide range of product categories. While the focus will be on drugs, the course will also touch upon medical devices, digital health, big data in health, medical apps, biomarkers, medical marketing, treatment guidelines, screening tools and diagnostics. Understanding clinical trial design and the regulatory pathway through the US FDA is a major focus of the course. Reimbursement is introduced for both drugs and medical devices. Each year, this course is usually able to negotiate some major project opportunities from teaching hospitals students can tackle, to expose them to the clinical world, an important target customer environment of pharmaceutical companies.
BTC1820H › Biotechnology in Agriculture & Natural Products {Year 1}
Session: Fall
Instructor: Duncan Jones
Credits: 0·5 R
Course Description:
This course will focus on the exploration and understanding of biotechnology as applied to agriculture, natural products, biocontrols and associated industrial biotechnology. Students will work in teams and each team will present their assigned topics as oral presentations and written assignments. A number of written individual assignments plus an exam will also be evaluated. In the agriculture area, lecture topics include modern approaches to plant breeding, genetically modified organisms (GMOs) and the controversy surrounding them; genomics and its importance in agribiotechnology; nutraceuticals; the use of natural and engineered products for pest and herbicide control; and the use of plants as bioreactors. In the natural products/biocontrols/industrial biotechnology areas, topics include the use of natural plant products for medicinal purposes; bioremediation of contaminated soils and the applications of biocatalysts as part of the green chemistry movement.
BTC2010H › Fundamentals of Managerial Concepts {Year 1}
Session: Fall
Instructor: Kevin Yousie
Credits: 0·5 R
Course Description:
This foundational course introduces students to a broad range of the critical managerial concepts that are required to operate successfully in today’s biotechnologically focused organisations. Topics covered include forms of business ownership, an introduction to financial statements, auditor reports, financial statement analysis, ratio analysis, time value of money, net present value, internal rate of return, projected statements, marketing math, market segmentation, product positioning, the marketing mix, pricing decisions, channel strategies, customer value propositions, competitive strategies, marketing in the age of artificial intelligence, as well as some aspects of strategic management and organisational alignment. Theory and application are combined through the use of readings, case studies, in-class discussions and presentations, as well as a team project.
BTC1600H › Biopartnering I {Year 1}
BTC1610H › Biopartnering II {Year 2}

Session: Fall
Instructor: Stephen Mac
Credits: 0·5 R
Course Description:
The ‘Biopartnering’ seminar series is a program requirement for all MBiotech students — in both the BioPh and DHT streams. BTC1600H and BTC1610H are held in conjunction with one another, meaning all students (regardless of year or program stream) attend the seminar on the same date and time. The seminar is held once per week during the Fall semester, on Tuesday evenings for approximately two hours. It is comprised of both presentations by select speakers from industry as well as student presentations. The course challenges students to provide insights into industry issues that would be seen as a valuable contribution by experts in the area. Each student will participate in a formal group presentation, in their first year, and will complete other academic requirements such as critiques, team mentoring and an individual report in their senior year. The topics presented in this course will range from scientific (latest technologies and research, analysis of pre-clinical and clinical data) to business-oriented issues (e.g., market strategies for pharma and biotechnology products, government regulations, intellectual property, finance, ethics, etc.)
› Jump to the Biopartnering Seminar page.
BTC2020H › Society, Organisations & Technology {Year 1}
Session: Fall
Instructor: Paul Chipperton
Credits: 0·5 R
Course Description:
This course introduces students to the fundamentals of economics and strategic management. Throughout the course, we will attempt to answer a fundamental question posed by management scholars: how is it that some firms are able to repeatedly and consistently achieve great results, while others fail and crash out of the market? In search of an answer, we’ll explore a variety of decisions firms make, including pricing, product variety and scope, motivation of employees, and interaction with competitors. The course features a combination of lecture and case discussion; course readings include textbook excerpts, business press, and academic articles.
BTC1900Y / 1910Y / 1920Y › Work Terms I, II & III {Year 1 + Year 2}

Preparation: Summer & Fall
Session: Year-Long (begins Winter)
Instructor: Kinza Khan
Credits: 1·0 R + 1·0 R + 1·0 E
Course Description:
This series of centrepiece courses is designed to grant our students a more in-depth appreciation and understanding of the biotechnology and biopharmaceutical industry in a corporate and/or industrial setting, and to apply their knowledge and skills in a real-world context. Students are required to complete two 4-month full-time Work Term placements that are arranged by the course coordinator to ensure that the role, responsibilities and activities are at a graduate level. Credit-granting responsibilities reside with the course instructor, based on assignments and feedback from students’ Work Term supervisors.
Required preparatory exercises and assignments must be completed in the Summer and Fall sessions, leading up to the start of the placement in Winter/Spring, in order for students to qualify for Work Term I. These preparatory requirements can involve résumé workshops, one-on-one meetings with members of the MBiotech team, attendance at the annual Career Day, and more.
Students in Work Term II may continue with the same employer from Work Term I, or alternatively with a new employer or department. Students’ performance and their work experiences is evaluated in a manner similar to that for Work Term I.
Note: BTC1920Y, Work Term III is an optional elective course that extends the placement experience to a full 12 months’ duration. Students taking Work Term III share their experiences by means of a mandatory Networking Night component in late November.
Students receive credit/no credit grades for all three courses.
BTC2030H › Management of Technological Innovation {Year 2}

Session: Winter
Instructor: Ruben Gaetani
Credits: 0·5 R
Course Description:
In this course, we will define technological innovation as the process of leveraging new ideas to create economic value and deliver this value to shareholders, employees, consumers, and our society at large. This process involves critical strategic choices that are common to most organisations, from small startups to large established companies: What is the best way to bring an idea to the market, and to arrange production and distribution? How should we redesign our internal organisation, as well as the system of partnerships and relationships with external players? Should we redefine our vertical and horizontal boundaries, for example by outsourcing some activities or entering new geographical markets? Throughout the course, we will refine our ability to approach and find the best answer to these (and many other) questions. Using an applied and discussion-based method, we will learn how to effectively convert a creative idea into a valuable innovation.
Since this Year 2 course runs concurrently with Work Terms, special remote learning provision is made available to those students who are out-of-province during the term, pending the Directors’ approval.
