New findings from UofT Mississauga highlight how enzymes make use of the empty binding site to drive a significant chemical reaction
A single carbonic anhydrase enzyme molecule can forge almost a million carbonate molecules from the substrate, carbon dioxide, every second. This might not mean a lot to most people, but we can be grateful this process occurs: we wouldn’t be able to breathe oxygen without it.
This area of research is of particular interest for Professor Scott Prosser and recent PhD graduate, Tae Hun Kim, from UTM’s Department of Chemical and Physical Sciences (CPS), who have just published their findings in the journal Science with co-authors in Biochemistry Emil Pai and student Pedram Mehrabi in the article, “The role of dimer asymmetry and protomer dynamics in enzyme catalysis.”
“This was the result of six years of intensive effort in both Emil’s lab and in my lab,” says Prosser, a prof in CPS.
“Our hope was to be able to describe the processes that take place in enzyme catalysis in terms of an “ensemble” (family) of structures and literally understand the dynamic changes that the enzyme undergoes to drive the reaction forward."
Enzymes, of which the vast majority are proteins, catalyze a myriad of processes in living systems. It turns out that nature tends to package many enzymes as pairs or dimers, begging the question, “Why?”
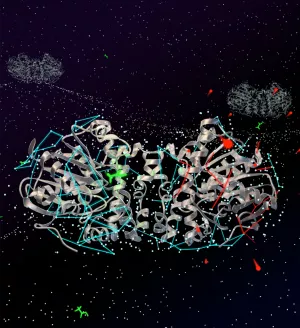
In many cases, dimeric enzymes exhibit cooperativity – that is, after binding the first substrate in the dimer, binding and turnover of the second substrate becomes even faster. Prosser and his research team recently uncovered another reason for the dimer in their study of an obscure bacterial enzyme, fluoroacetate dehalogenase. The role of this dimeric enzyme is to pry away a fluorine atom from an otherwise potent and lethal pesticide, fluoroacetate. This is no small feat: the carbon-fluorine bond is one of the strongest chemical bonds in nature.
Through a technique called X-ray crystallography, in combination with a spectroscopic method called Nuclear Magnetic Resonance (NMR), the research team was able to assemble a series of high-resolution snapshots along with dynamic information on the dimeric enzyme associated with various stages of catalysis, which is the process of a chemical reaction created by an additional substance that serves as the catalyst.
The team also found that water molecules aren’t just used by the enzyme as “ballast” to drive the reaction. Very subtle networks of bound water molecules distinguish the enzyme at each step along the reaction pathway.
Prosser says that these findings are leading to the discovery that bound water networks are more involved in protein function than scientists had previously imagined.
